The Gift To The World – Coming November 11 2020
Eddie’s Announcement June 9 2020
The Gift To The World – Is Here
Open-Source Project
As promised, the gift to the world is now available to every man woman and child on planet earth.
Our planet has been systematically poisoned, polluted, burnt and depleted from her precious gifts. Our children will live in an imbalanced environment unless we take action on a scale that has never been seen before. The most pivotal year of human history exposed the depths of this sinister behavior. 2020 will go down in history as the year humanity was “shaken” into an awakening that led us on the path to true freedom.
This path requires us to come together – The Family of Planet Earth – UNITED.
We have the resources, ideas and will to build, clean, revive and correct every part of our environment that is screaming for help.
There are many around the world who are great innovators, inventors self-taught inventors. Some tried to bring their valuable innovation to life but were kept shut because their idea was simply too good. Those who were responsible for the sinister behavior were “ended” every idea that could potentially be in the way of their Plan.
Our path ahead is not a reflection of the past, but a reality we are consciously creating and, aware of its outcome. We are free and safe to unravel the gifts we have for the world. To speak out and share with the world what we can offer and how we can help.
Today November 11 2020, I offer to the world an idea, a concept. This may have been thought of prior, what’s important is that this idea is ready for you all.
I hope, that my step will evoke something. I don’t know what or how but, what I do know it will be beautiful and rewarding to all.
With love,
Eddie BenAbraham
Inventor, President and Co Founder @ Vort8x
I Want to Collaborate - Gift To The World
LIN Well Power Plant
This “Original Plan” was written by Eddie BenAbraham in 2011. Eddie owns the content. This concept/idea is for the entire world. This content is not for sale or to be bartered with.
IT IS COMPLETELY AND ABSOLUTELY FREE.
LIN Well Power Plant
The first docs
By
Inventor & Designer
Eddie BenAbraham
December 19th 2011
– The concept
– Technology involved
– Overall steps and objectives
– Testing
– Pros and Cons
– Drilling and location
– Permits and bureaucracy
– Cost projections
– ROI
– Diagrams
– Understanding “N” and “LN2”
– Additional Info
The Concept
Geothermic activity on the earth’s crust has enabled us to extract its potentials on a very limited basis. It is not a new concept nor unfamiliar nevertheless, it is in need of a new paradigm.
The ideas and possibilities which I am presenting in this plan are new and revolutionary because it will enable us to create electrical energy at many more locations ever thought possible, much safer than any other industrial size power plant using nuclear or fossil fuels.
Thanks to our technological advancements in recent years we will be able to implement the proposed idea with relative ease and at realistic costs.
Today’s generation of electrical energy using the heat of mother earth is consumed with limitations and risks which cannot be ignored. In order to attain the steam flow and pressure needed to turn a turbine, water must reach temperatures exceeding 200 degrees Celsius. Such conditions are limited to geo-thermaly active locations and in extreme depths at non active locations. Both raise a common and leading risk of triggering earthquakes and then the other challenges such as costs and availability of nearby water supply.
Imagine the possibility of drilling only a fraction of the depth almost anywhere on earth and reaching high steam pressure enabling the turning of small to medium scale turbines.
The concept I am proposing involves using a substance/element other than water.
This substance is available abundantly on earth and it is safe!
It is Liquid Nitrogen LN2 or LIN
The element Nitrogen “N” comes as the first candidate for achieving this mission’s goal. It is safe, abundant and the one of its main characteristics is its low boiling point. Liquid nitrogen (LIN) can be produced at relatively low costs which adds an additional advantage in pursuing this route.
With Liquid nitrogen we will only need to reach 30-40 degrees Celsius in order to achieve a reaction needed to accommodate the standard specifications of a commercially available turbine and electrical generator system. Such temperature can be obtained very easily at depths no more than one mile which is safe and can be attained at much lower cost.
Green, Safe and Cheap energy production is at the core of this plan and there is no doubt in my mind it will become one of the leading technological breakthroughs which will allow a calm transition away from the old polluting and unsafe of fossil and nuclear generation.
Technology involved
A- Liquid Nitrogen LIN, LN2
Turning Nitrogen gas into liquid is a process well known and practiced for over 100 years, it is simple yet it does require the proper instruments and energy.
Nitrogen gas is the most abundant element in our earth’s atmosphere, about 78%, which makes it practical and available all around. By compressing and cooling ambient air below 77K (-196 °C; -321 °F) Nitrogen (N) is turned into Liquid Nitrogen (LIN,LN2).
With advancements in science, this basic process has been improved to production rates in excess of 200 tons per day on a commercial scale. There are smaller and compact LIN Generators available for small and medium size needs.
Our objective will be to calculate the average daily consumption of the LIN Well Power Plant in order to install the best suited LN2 generator.
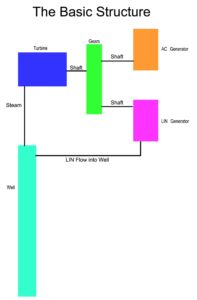
B- Steam Turbine and Generator
Steam driven turbines are an advanced science these days, as a result of more and more efficient aircraft turbine engines reaching pinnacle stages of efficiency and reliability. Based on our objectives, more than a few options are available and ready to purchase.
Attached are details of a few set-ups which are in my output objective range of 1000-3000psi steam Well output.
Siemens offers a range of steam turbines anywhere from 45 kW to 1,200 MW,with the flexibility to address a few specific requirements.
A unit such as the SST-300 will enable us a production of up to 50mw of power however, the requirements are for 1740psi of inlet steam. This means based on the estimates, efficiency and capability of LIN generators to produce the amount needed, along with the efficiency and integrity of the apparatus, it will be possible to conclude which turbine/generator setup is best.
C- Apparatus
The apparatus job is to:
- Contain the high pressure.
- Direct the high pressure.
- Have efficient temperature conductivity.
- House the LIN insertion mechanism
- Carry diagnostic instrumentation.
These are the main but not all the characteristics and features required for a safe and efficient plant operation. It will therefore, be one of the main first tests to conduct on a small setup which will help determine the first operational large scale design.
Depending on the drilling capabilities of the industry, there maybe limitations and guidelines I will need to consider.
D- Insertion Mechanism
The apparatus will contain Nitrogen pressure in excess of 3000psi which offers a challenge in the method of replenishing the Well with fresh LIN. Pumping the LIN into the well will consume energy which can possibly reduce the plant efficiency however, a simple 3 stage mechanical valve system will perform the necessary task using less energy and at acceptable efficiency.
Overall steps and objectives
Stage #1
-Step
Testing LN2 in different apparatus designs on a small scale.
-Objective
Conclude the most efficient design and material to use for industrial scale generation of electrical power. This will co-inside with the capabilities of today’s drilling in terms of size of drill and underground links and creation of artificial caverns or extensions.
-Step
Comparing available electrical generation systems from the stage of the turbine to generator to grid output.
-Objective
Conclude the type of turbine-generator-gear combination most effective and needed instruments based on the generated pressure, temperature and other predominant characteristics as a result of using LN2.
Generator output must meet our objectives which are high efficiency with a target input pressure of 1000-3000psi.
-Step
Conduct ground surveys at potential locations
-Objectives
Find most probable locations for a 1.5 Mlle depth max with potential ground temperature exceeding 35 degrees Celsius.
Locating most favorable districts and or counties, states that will be willing to corporate extensively.
One option to consider is leasing or buying a location with an old drilled water or gas well to allow savings in drilling costs.
-Step
Liquid nitrogen generators cost, volume and efficiency.
-Objective
Large quantities will be needed to operate a sizable power plant therefore the efficiency and speed of such mechanism must be at optimum. Also conclude if connecting the LIN generator to the mechanical output of the well would be more efficient. As a consequence, liquid oxygen is generated during the same process of making LIN which can offer and additional element for use in powering the Plant.
-Step
Cost projections
-Objective
Gather detailed foreseeable costs of all aspects of the project along with the cost of production.
Looking ahead in order to foresee possible variables the project might face.
Testing
Part 1
A- Primal testing will be done with scaled down system in order to observe and conclude the best structure design and parts for the Well.
Considering all pieces to this puzzle there are 2 with high priority: 1- The apparatus, shape, scale and material. 2- Directly linked to #1 is the ratio of LN2 needed to feed the system in order to keep a constant of safe usable steam.
B- Turbines are built and designed for high temperature steam intake, our job will be to make sure proper testing has been done by the manufacturer at low temperatures in order to avoid failure of material and turbine integrity.
C- Using the prototype, test the locations best suited for a fail-safe devices in the event of a possible failure scenario. The LIN Well Power Plant will not contain or use harmful chemicals however, it will create high steam pressure using LN2 which can become unstable at certain conditions if precautions are not properly addressed and or implemented.
Part 2 (Post drill)
A- Physics offers us the best possible results however, discovering the quantity and flow ratio of LIN will need to be concluded on a real well. As a second stage step it will be our required and concluding test before a final choice of turbine and generator set up is made. At the same time, pressure sustainability and safety tests will be conducted.
Pros and Cons
Pros:
1- Green!! no impact on the environment
2- No toxic or radioactive materials required or expelled.
3- Can be installed in many location.
4- Relatively small in structural size
5- Only shallow drilling required to reach 30-40 degrees celsius (about ¾ – 1.5 mile depending on location).
6- LIN Well Plant farm is doable.
7- LIN is very cheap to manufacture
Cons:
1- Each LIN Well will generate relatively lower output than one Coal, Nuclear or a large Hydroelectric plant.
2- Never been tested on a small or large scale.
3- Medium size Initial cost.
4- Liquid nitrogen generators output and efficiency need to improve.
Drilling and location
Location will be a relatively flexible matter since our objective temperature can be achieved by drilling in many locations around the earth to depths not exceeding 1.5 mile.
A cost issue arises which can top this project out of a reasonable budget therefore, I propose looking into used/old drilled sites that can suite our requirements of:
-Depth
-Temperature
-Location
-Co-operation of local authorities
-Vicinity to populated areas (to sell the power to)
Looking into well known active drillers states with a relative low land cost will be a priority such as Texas. Two more factors need to be taken into consideration, one- is which location has “softer” laws and two- is a reasonable geothermal gradient which averages about 22.1°C per km of depth (1°F per 70 feet of depth) in most of the world, which means some locations can be lower or higher therefore earlier statistics and tests are needed (See additional details in following pages)
Permits and Bureaucracy
Will be addressed during location hunt.
Cost & Projections
A- Initial
- Physicist
- Chemist
- Drilling Expert
B- Site and drilling cost
- Survey
- Purchase/Lease
- Permits
- Drilling
C- Equipment cost
- LIN generator
- Apparatus (custom pipe)
- Turbine & Generator
- Transformers
- Control station
- Transmission towers
- Insertion mechanism
- Other parts
D- Construction cost
- Foundations
- Structure
- Road
- Gates
- Security
- Other
D- Testing cost
- Materials
- Location
E- Operation and production cost-
- Employees
- Replacement parts
- Lease or land costs
- License and permits
ROI
The tall order of this project is to have a self sustaining power plant with a long operational life span. This goal along with reasonable R&D and construction costs, will offer a lower levelized cost than any other conventional power plant in use today.
Because of its characteristics, the return on the LIN Well Power Plant will be significantly large and very attractive.
Designing and building only one Plant is definitely not on the agenda but quite the opposite. Once the first Plant is operational, the objective will be to make LIN Well Power Plants available to all around the world.
Such an operation can generate large cash-flow for a considerable length of time moreover, patents and exclusivity rights will offer great advantages as well.
I estimate a year to 18 months at the most from the time of testing to a commercially viable LIN Well Power Plant.
Diagrams
Lin Well Power Plant
Binary Geothermal Power Plant
(image removed copyright)
Vertical and Horizontal Wells
(image removed copyright)
Understanding “N” and “LN2”
Nitrogen “N”
a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth’s atmosphere.[1] The element nitrogen was discovered as a separable component of air, by Scottish physician Daniel Rutherford, in 1772.
Many industrially important compounds, such as ammonia, nitric acid, organic nitrates (propellants and explosives), and cyanides, contain nitrogen. The extremely strong bond in elemental nitrogen dominates nitrogen chemistry, causing difficulty for both organisms and industry in breaking the bond to convert the N2 into useful compounds, but at the same time causing release of large amounts of often useful energy when the compounds burn, explode, or decay back into nitrogen gas.
Nitrogen occurs in all living organisms, and the nitrogen cycle describes movement of the element from the air into the biosphere and organic compounds, then back into the atmosphere. Synthetically produced nitrates are key ingredients of industrial fertilizers,[1] and also key pollutants in causing the eutrophication of water systems. Nitrogen is a constituent element of amino acids and thus of proteins and nucleic acids (DNA and RNA). It resides in the chemical structure of almost all neurotransmitters, and is a defining component of alkaloids, biological molecules produced by many organisms. The human body contains about 3% by weight of nitrogen, a larger fraction than all elements save oxygen, carbon, and hydrogen.
Preparation
Commercially nitrogen is produced by fractional distillation of air. In a chemical laboratory it is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
NH4Cl(aq) + NaNO2(aq) —> N2(g) + NaCl(aq)
Small amounts of impurities NO and HNO3 are also formed in this reaction. The impurities can be removed by passing the gas through aqueous sulfuric acid containing potassium dichromate.
Very pure nitrogen can be prepared by the thermal decomposition of barium or sodium azide.
Na(N3)2 + heat —> Na + 3N2
Properties
Nitrogen is a nonmetal, with an electronegativity of 3.04. It has five electrons in its outer shell and is, therefore, trivalent in most compounds. The triple bond in molecular nitrogen (N2) is one of the strongest. The resulting difficulty of converting N2 into other compounds, and the ease (and associated high energy release) of converting nitrogen compounds into elemental N2, have dominated the role of nitrogen in both nature and human economic activities.
At atmospheric pressure molecular nitrogen condenses (liquefies) at 77 K (−195.79 °C) and freezes at 63 K (−210.01 °C)[1] into the beta hexagonal close-packed crystal allotropic form. Below 35.4 K (−237.6 °C) nitrogen assumes the cubic crystal allotropic form (called the alpha-phase). Liquid nitrogen, a fluid resembling water in appearance, but with 80.8% of the density (the density of liquid nitrogen at its boiling point is 0.808 g/mL), is a common cryogen.
Unstable allotropes of nitrogen consisting of more than two nitrogen atoms have been produced in the laboratory, like N3 and N4.[6] Under extremely high pressures (1.1 million atm) and high temperatures (2000 K), as produced using a diamond anvil cell, nitrogen polymerizes into the single-bonded cubic gauche crystal structure. This structure is similar to that of diamond, and both have extremely strong covalent bonds. N4 is nicknamed “nitrogen diamond.”[7]
Other (as yet unsynthesized) allotropes include hexazine (N6, a benzene analog)[8] and octaazacubane (N8, a cubane analog).[9] The former is predicted to be highly unstable, while the latter is predicted to be kinetically stable, for reasons of orbital symmetry.[10]
Electromagnetic spectrum
Nitrogen discharge (spectrum) tube
Molecular nitrogen (14N2) is largely transparent to infrared and visible radiation because it is a homonuclear molecule and, thus, has no dipole moment to couple to electromagnetic radiation at these wavelengths. Significant absorption occurs at extreme ultraviolet wavelengths, beginning around 100 nanometers. This is associated with electronic transitions in the molecule to states in which charge is not distributed evenly between nitrogen atoms. Nitrogen absorption leads to significant absorption of ultraviolet radiation in the Earth’s upper atmosphere and the atmospheres of other planetary bodies. For similar reasons, pure molecular nitrogen lasers typically emit light in the ultraviolet range.
Nitrogen also makes a contribution to visible air glow from the Earth’s upper atmosphere, through electron impact excitation followed by emission. This visible blue air glow (seen in the polar aurora and in the re-entry glow of returning spacecraft) typically results not from molecular nitrogen but rather from free nitrogen atoms combining with oxygen to form nitric oxide (NO).
Nitrogen gas also exhibits scintillation.
Reactions
In general, nitrogen is unreactive at standard temperature and pressure. N2 reacts spontaneously with few reagents, being resilient to acids and bases as well as oxidants and most reductants. When nitrogen reacts spontaneously with a reagent, the net transformation is often called nitrogen fixation.
Nitrogen reacts with elemental lithium.[12] Lithium burns in an atmosphere of N2 to give lithium nitride:
6 Li + N2 → 2 Li3N
Magnesium also burns in nitrogen, forming magnesium nitride.
3 Mg + N2 → Mg3N2
N2 forms a variety of adducts with transition metals. The first example of a dinitrogen complex is [Ru(NH3)5(N2)]2+ (see figure at right). Such compounds are now numerous, other examples include IrCl(N2)(PPh3)2, W(N2)2(Ph2PCH2CH2PPh2)2, and [(η5-C5Me4H)2Zr]2(μ2, η2,η2-N2). These complexes illustrate how N2 might bind to the metal(s) in nitrogenase and the catalyst for the Haber process.[13] A catalytic process to reduce N2 to ammonia with the use of a molybdenum complex in the presence of a proton source was published in 2005.[12]
The starting point for industrial production of nitrogen compounds is the Haber process, in which nitrogen is fixed by reacting N2 and H2 over an iron(III) oxide (Fe3O4) catalyst at about 500 °C and 200 atmospheres pressure. Biological nitrogen fixation in free-living cyanobacteria and in the root nodules of plants also produces ammonia from molecular nitrogen. The reaction, which is the source of the bulk of nitrogen in the biosphere, is catalyzed by the nitrogenase enzyme complex that contains Fe and Mo atoms, using energy derived from hydrolysis of adenosine triphosphate (ATP) into adenosine diphosphate and inorganic phosphate (−20.5 kJ/mol).
Occurrence
Nitrogen is the largest single constituent of the Earth’s atmosphere (78.082% by volume of dry air, 75.3% by weight in dry air). It is created by fusion processes in stars, and is estimated to be the seventh most abundant chemical element by mass in the universe.[14]
Molecular nitrogen and nitrogen compounds have been detected in interstellar space by astronomers using the Far Ultraviolet Spectroscopic Explorer.[15] Molecular nitrogen is a major constituent of the Saturnian moon Titan’s thick atmosphere, and occurs in slightly appreciable to trace amounts in other planetary atmospheres.[16]
Nitrogen is present in all living organisms, in proteins, nucleic acids, and other molecules. It typically makes up around 4% of the dry weight of plant matter, and around 3% of the weight of the human body. It is a large component of animal waste (for example, guano), usually in the form of urea, uric acid, ammonium compounds, and derivatives of these nitrogenous products, which are essential nutrients for all plants that cannot fix atmospheric nitrogen.
Nitrogen occurs naturally in many minerals, such as saltpetre (potassium nitrate), Chile saltpetre (sodium nitrate) and sal ammoniac (ammonium chloride). Most of these are uncommon, partly because of the minerals’ ready solubility in water. See also Nitrate minerals and Ammonium minerals.
Production and applications
Nitrogen gas is an industrial gas produced by the fractional distillation of liquid air, or by mechanical means using gaseous air (i.e., pressurized reverse osmosis membrane or Pressure swing adsorption). Commercial nitrogen is often a byproduct of air-processing for industrial concentration of oxygen for steelmaking and other purposes. When supplied compressed in cylinders it is often called OFN (oxygen-free nitrogen).[17]
Nitrogen gas has a variety of applications, including serving as an inert replacement for air where oxidation is undesirable;
As a modified atmosphere, pure or mixed with carbon dioxide, to preserve the freshness of packaged or bulk foods (by delaying rancidity and other forms of oxidative damage)
In ordinary incandescent light bulbs as an inexpensive alternative to argon.[18]
The production of electronic parts such as transistors, diodes, and integrated circuits
Dried and pressurized, as a dielectric gas for high-voltage equipment
The manufacturing of stainless steel[19]
Used in military aircraft fuel systems to reduce fire hazard, (see inerting system)
On top of liquid explosives as a safety measure
Filling automotive and aircraft tires[20] due to its inertness and lack of moisture or oxidative qualities, as opposed to air. The difference in N2 content between air and pure N2 is 20%[21][22]
Used as a propellant for draught wine, and as an alternative to or together with carbon dioxide for other beverages.
Nitrogen is commonly used during sample preparation procedures for chemical analysis. It is used to concentrate and reduce the volume of liquid samples. Directing a pressurized stream of nitrogen gas perpendicular to the surface of the liquid allows the solvent to evaporate while leaving the solute(s) and un-evaporated solvent behind.[23]
Nitrogen tanks are also replacing carbon dioxide as the main power source for paintball guns. Nitrogen must be kept at higher pressure than CO2, making N2 tanks heavier and more expensive.
Safety
Rapid release of nitrogen gas into an enclosed space can displace oxygen, and therefore represents an asphyxiation hazard. This may happen with few warning symptoms, since the human carotid body is a relatively slow and a poor low-oxygen (hypoxia) sensing system.[35] An example occurred shortly before the launch of the first Space Shuttle mission in 1981, when two technicians lost consciousness (and one of them died) after they walked into a space located in the Shuttle’s Mobile Launcher Platform that was pressurized with pure nitrogen as a precaution against fire. The technicians would have been able to exit the room if they had experienced early symptoms from nitrogen-breathing.
When inhaled at high partial pressures (more than about 4 bar, encountered at depths below about 30 m in scuba diving), nitrogen begins to act as an anesthetic agent. It can cause nitrogen narcosis, a temporary semi-anesthetized state of mental impairment similar to that caused by nitrous oxide.[36][37]
Nitrogen also dissolves in the bloodstream and body fats. Rapid decompression (in particular, in the case of divers ascending too quickly, or astronauts decompressing too quickly from cabin pressure to spacesuit pressure) can lead to a potentially fatal condition called decompression sickness (formerly known as caisson sickness or the bends), when nitrogen bubbles form in the bloodstream, nerves, joints, and other sensitive or vital areas.[38][39] Other “inert” gases (those gases other than carbon dioxide and oxygen) cause the same effects from bubbles composed of them, so replacement of nitrogen in breathing gases may prevent nitrogen narcosis, but does not prevent decompression sickness.[40]
Direct skin contact with liquid nitrogen will cause severe frostbite (cryogenic “burns”). This may happen almost instantly on contact, or after a second or more, depending on the form of liquid nitrogen. Bulk liquid nitrogen causes less rapid freezing than a spray of nitrogen mist (such as is used to freeze certain skin growths in the practice of dermatology). The extra surface area provided by nitrogen-soaked materials is also important, with soaked clothing or cotton causing far more rapid damage than a spill of direct liquid to skin. Full “contact” between naked skin and large collected-droplets or pools of liquid nitrogen may be prevented for a second or two, by a layer of insulating gas from the Leidenfrost effect. This may give the skin a second of protection from nitrogen bulk liquid. However, liquid nitrogen applied to skin in mists, and on fabrics, bypasses this effect, and causes local frostbite immediately.
Liquid Nitrogen “LN2”
is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4.[dead link][1] Liquid nitrogen is often referred to by the abbreviation, LN2 or “LIN” or “LN” and has the UN number 1977.
At atmospheric pressure, liquid nitrogen boils at 77K (-196°C; -321°F) and is a cryogenic fluid which can cause rapid freezing on contact with living tissue, which may lead to frostbite. When appropriately insulated from ambient heat, liquid nitrogen can be stored and transported, for example in vacuum flasks. Here, the very low temperature is held constant at 77 K by slow boiling of the liquid, resulting in the evolution of nitrogen gas. Depending on the size and design, the holding time of vacuum flasks ranges from a few hours to a few weeks.
Liquid nitrogen can easily be converted to the solid by placing it in a vacuum chamber pumped by a rotary vacuum pump.[2] Liquid nitrogen freezes at 63 K (−210 °C; −346 °F). Despite its reputation, liquid nitrogen’s efficiency as a coolant is limited by the fact that it boils immediately on contact with a warmer object, enveloping the object in insulating nitrogen gas. This effect, known as the Leidenfrost effect, applies to any liquid in contact with an object significantly hotter than its boiling point. More rapid cooling may be obtained by plunging an object into a slush of liquid and solid nitrogen than into liquid nitrogen alone.
Nitrogen was first liquefied at the Jagiellonian University on 15 April 1883 by Polish physicists, Zygmunt Wróblewski and Karol Olszewski.
Uses
Liquid nitrogen is a compact and readily transported source of nitrogen gas without pressurization. Further, its ability to maintain temperatures far below the freezing point of water makes it extremely useful in a wide range of applications, primarily as an open-cycle refrigerant, including:
in cryotherapy for removing unsightly or potentially malignant skin lesions such as warts and actinic keratosis
as a coolant for CCD cameras in astronomy
to store cells at low temperature for laboratory work
in cryogenics
as a source of very dry nitrogen gas
for the immersion freezing and transportation of food products
for the cryopreservation of blood, reproductive cells (sperm and egg), and other biological samples and materials
as a method of freezing water pipes in order to work on them in situations where a valve is not available to block water flow to the work area
in the process of promession, a way to dispose of the dead
for cooling a high-temperature superconductor to a temperature sufficient to achieve superconductivity
for the cryonic preservation in the hope of future reanimation.
to preserve tissue samples from surgical excisions for future studies
to shrink-weld machinery parts together
as a coolant for vacuum pump traps and in controlled-evaporation processes in chemistry.
as a coolant to increase the sensitivity of infrared homing seeker heads of missiles such as the Strela 3
as a coolant to temporarily shrink mechanical components during machine assembly and allow improved interference fits
as a coolant for computers[4]
in food preparation, such as for making ultra-smooth ice cream.[5]
Safety
Since the liquid to gas expansion ratio of nitrogen is 1:694 at 20C, a tremendous amount of force can be generated if liquid nitrogen is rapidly vaporized. In an incident in 2006 at Texas A&M University, the pressure-relief devices of a tank of liquid nitrogen were malfunctioning and later sealed. As a result of the subsequent pressure buildup, the tank failed catastrophically and exploded. The force of the explosion was sufficient to propel the tank through the ceiling immediately above it.[6]
Because of its extremely low temperature, careless handling of liquid nitrogen may result in cold burns.
As liquid nitrogen evaporates it will reduce the oxygen concentration in the air and might act as an asphyxiant, especially in confined spaces. Nitrogen is odorless, colorless and tasteless, and may produce asphyxia without any sensation or prior warning.[7] A laboratory assistant died in Scotland in 1999, apparently from asphyxiation, possibly caused by liquid nitrogen spilled in a basement storage room.[8]
Vessels containing liquid nitrogen can condense oxygen from air. The liquid in such a vessel becomes increasingly enriched in oxygen (boiling point = 90 K) as the nitrogen evaporates, and can cause violent oxidation of organic material.[citation needed]
Additional info
Geothermal Gradient
The geothermal gradient is the rate of change of temperature (ΔT) with depth (ΔZ), in the earth. Units of measurement are °F/100 ft or °C/km. In the geosciences, the measurement of T is strongly associated with heat flow, Q, by the simple relation: Q=KΔT/ΔZ, where K is the thermal conductivity of the rock.
Temperatures at the surface of the earth are controlled by the Sun and the atmosphere, except for areas such as hot springs and lava flows. From shallow depths to about 200 ft (61 m) below the surface, the temperature is constant at about 55°F (11°C). In a zone between the near surface and about 400 ft (122 m), the gradient is variable because it is affected by atmospheric changes and circulating ground water. Below that zone, temperature almost always increases with depth. However, the rate of increase with depth (geothermal gradient) varies considerably with both tectonic setting and the thermal properties of the rock.
High gradients (up to 11°F/100 ft, or 200°C/km) are observed along the oceanic spreading centers (for example, the Mid-Atlantic Rift) and along island arcs (for example, the Aleutian chain). The high rates are due to molten volcanic rock (magma) rising to the surface. Low gradients are observed in tectonic subduction zones because of thrusting of cold, water-filled sediments beneath an existing crust. The tectonically stable shield areas and sedimentary basins have average gradients that typically vary from 0.82–1.65°F/100 ft (15–30°C/km).
Measurements of thermal gradient data in Japan range widely and over short horizontal distances between to 0.6–4.4°F/100 ft (10–80°C/km). The Japanese Islands are a volcanic island arc that is bordered on the Pacific side by a trench and subduction complex. The distribution of geothermal gradients is consistent with the tectonic settings. In the northeastern part of Japan, the thermal gradient is low on the Pacific side of the arc and high on the back-arc side. The boundary between the outer low thermal gradient and the high thermal gradient regions roughly coincides with the boundary of the volcanic front.
The geothermal gradient is important for the oil, gas, and geothermal energy industries. Downhole logging tools must be hardened if they are to function in deep oil and gas wells in areas of high gradient. Calculation of geothermal gradients in the geological past is a critical part of modeling the generation of hydrocarbons in sedimentary basins. In Iceland, geothermal energy, the main source of energy, is extracted from those areas with geothermal gradients ≥2.2°F/100 ft (≥40°C/km).
History
A BRIEF HISTORY OF GEOTHERMAL POWER
GENERATION
Ninety-five years ago, in the Tuscany village of
Larderello, electricity first flowed from geothermal energy
when Prince Piero Ginori Conti powered a 3/4-horsepower
reciprocating engine to drive a small generator. The Prince
was thereby able to light a few bulbs in his boric acid factory
situated amid the boron-rich geothermal steam field. He up-
graded the power system to 20 kW in 1905 [1].
Commercial delivery of geothermally-generated elec-
tric power occurred in 1914 when a 250 kW unit at Larderello
provided electricity to the nearby cities of Volterra and
Pomarance. Prior to being destroyed in 1944 during World
War II, Larderello had a total power capacity of 136,800 kW,
an annual generation greater than 900 GWh, and an average
annual capacity factor of more than 75 percent. The plants
were rebuilt after the war and extensive development of the
steam field began. Today, there are over 740 MW installed at
Larderello and the other nearby geothermal fields in the
Tuscany region of Italy. Many of the power plants are in the
15-25 MW range, qualifying them as small power plants.
New Zealand was the first country to operate a com-
mercial geothermal power plant using a liquid-dominated, hot-
water type reservoir (as contrasted with the steam-type at
Larderello). This took place at Wairakei in 1958. The United
States became the third country to use geothermal energy to
generate electricity in 1960 when the Pacific Gas & Electric
Company (PG&E) inaugurated an 11 MW Geysers Unit 1.
This small plant later earned the designation as a Mechanical
Engineering Historical Landmark. The U.S. has become the
largest generator of geothermal electricity with an installed
capacity of 2850 MW [2,3]. A summary of the state of world-
wide installed geothermal electric generating capacity is given
in Table 1 [4].
Cont:
http://geoheat.oit.edu/bulletin/bull20-2/art1.pdf
What is Levelized Cost?
Solar and renewable energy systems “fix” your energy cost in time: Once installed, it will provide years of energy — “Levelized cost” is the average cost of this renewable energy.
Levelized Cost = Net Cost to install a renewable energy system divided by its expected life-time energy output.
For example: If a solar energy system costs $10,000 to install (after all rebates) and it provides 100,000 kWh of electricity over its life, then the Levelized Cost of the solar energy system is $10,000 / 100,000 kWh = $0.10 per kWh
So, if you pay $0.10 today for your utility-supplied electricity, in this example, your solar electric cost is fixed at $0.10 / kWh — all the while the utility electricity that you would have purchased (if you did not install solar) will likely escalate over time (due to price inflation).
To print as supplemental
Turbines
energy cost outlook
http://205.254.135.7/tools/faqs/faq.cfm?id=19&t=3
Links
Evaporation rate at 40 Celsius – with video
http://www.edurite.com/kbase/evaporation-rate-of-liquid-nitrogen#
http://www.ormat.com/air-cooling
Cost of future power production in details
Measure Your Compassion
CLICK "VOTE", OUR SYSTEM WILL AUTOMATICALLY GAUGE YOUR STATE OF COMPASSION.
The objective is to find out how compassionate this healing event is.
For the first 5 seconds, you will see your own result before it is combined into a collective-average.
- Click once.
- No Personal Information is Needed.
- The Tool Detects Your Energy The Moment You "VOTE".
- The more compassion the subject evokes in you, the higher they will rank.
Click here to learn how it works
Why Measure Compassion?
Our goal is to promote compassionate actions in world communities. We offer the Compassion Gauge Tool on specific pages with the intent to inspire people and organizations to meld compassion into their mission and values.
The more compassion the subject evokes in you, the higher the results.
Eddie BenAbraham
President and co-founder
What If I Get Zero?
Does NOT mean you are not a compassionate person!
Compassion is a state-of-being just like love, anger or joy and for each state, there are different levels of experience.
Compassion – Marketing For A New Era – What Will You Choose?
Compassion measurement tool, Compassion measurement, Self compassion measure, Measuring compassion, Levels of compassion, How to measure compassion







